Our research is focused on understanding the coupling between chemistry and mechanics at material interfaces. The overarching motivation for this study of chemomechanics is the biological cell. There is increasing experimental evidence that changes in the local mechanical environment (e.g., material stiffness or applied force) and chemical environment (e.g., pH or biomolecule concentrations) of cells correlate with changes in cell shape and function. Although the individual proteins at this interface are now well studied, the mechanisms by which mechanical and chemical signals are exchanged across this interface to impact cell functions are not fully understood. We aim to elucidate this chemomechanical coupling at the molecular scale, leveraging the perspectives and tools of materials physics. We focus on cell interfaces and environments relevant to wound healing and inflammation, cancer, and stem/precursor cell development. To aid our development of new tools and models of such complex interfaces, we also study engineered nanocomposites and nanostructures that share this strong chemomechanical coupling.
We investigate material chemomechanics through both experiments and simulations, which are integrated among two main efforts:
Chemomechanics at the cell-material interface: Here, we seek to understand the mechanisms by which the mechanical and biochemical properties of extracellular microenvironments modulate cell adhesion and biological processes. Furthermore, we seek to leverage this understanding to develop improved ways to produce living cells as medicines to improve human health. For example:
- effect of substrata stiffness on the differentiation and migration of human oligodendrocytes (Espinosa-Hoyos et al., 2020)
- effect of mechanical strain on nuclear dynamics and cytoskeleton rearrangement (Makhija et al., 2018)
- effect of substrata viscoelastic properties on the secretome of mesenchymal stromal cells (Liu et al., 2018)
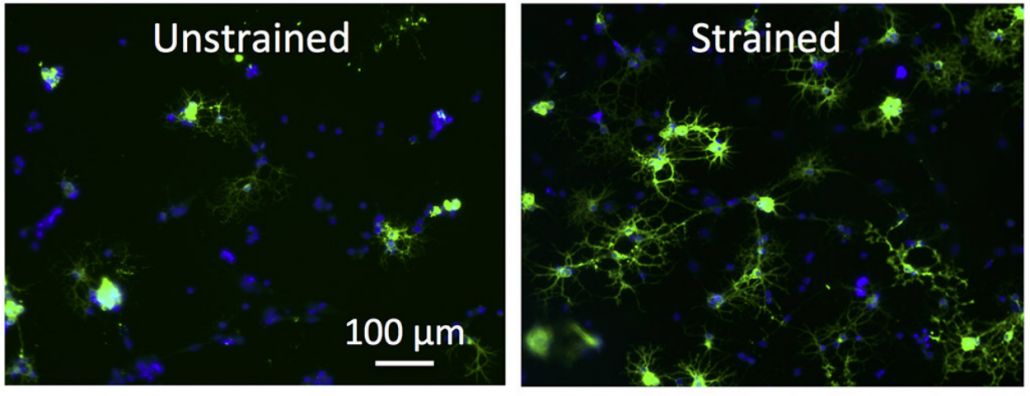
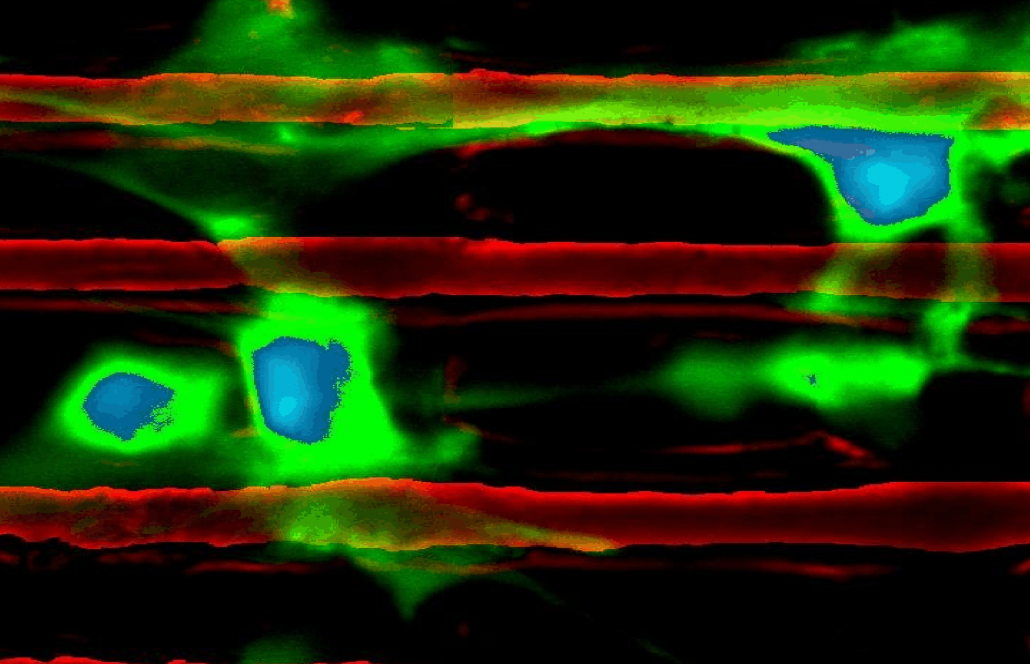
Chemomechanics of complex gels:
Both the interior and exterior of biological cells comprise materials described as crowded gels and polymer networks, often existing in metastable states that are perturbed via external cues. We explore chemomechanics in synthetic gels that either (1) interface with biological cells, and thus serve as a tool to modulate cell environments; or (2) are not intended as biomaterials, but serve as excellent physical models of such complex biopolymers. For example:
- engineering 3D-printed artificial axons that recapitulate the mechanical and geometric properties of CNS axons (Espinosa-Hoyos et al., 2018)
- developing synthetic gel composites that mimic the energy dissipation response of brain tissue (Qing et al., 2016)
- engineering mechanically tunable synthetic organogels as biofidelic tissue simulants (Kalcioglu et al., 2013)
Previous Focuses
Chemomechanics of nanocomposite interfaces:
The interfaces within subcellular structures – and also between cells & adjacent materials – are dynamic. Both biologists and material scientists have referred to these interfacial regions as interphases, characterized by unique nanostructure and viscoelastic behavior. Here, we develop new computational models and experiments to characterize the chemomechanical evolution of such nanoscale interphases in engineered materials. For example:
- atomic resolution imaging of nanoscale chemical Expansion in PrxCe1–xO2−δ during in situ heating (Swallow et al., 2018)
- decoupling mechanisms of fracture-induced performance degradation in LiXMn2O4 for Li-ion battery development (McGrogan et al., 2018)
- modeling the effects of biaxial strain on the concentration and mobility of electronic defects in SrTiO3(Chi et al., 2018)


